Arthrex Achieves First Case with NanoNeedle™ Scope 2.0
Milestone Pediatric Case Launches Next-Generation Visualization Using Minimally Invasive Scope
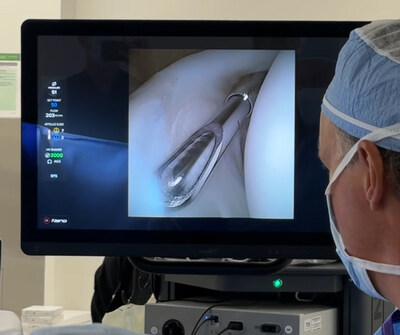
NAPLES, Fla., Sept. 23, 2025 /PRNewswire/ — Arthrex, a global leader in minimally invasive surgical technology, announced the launch of NanoNeedle™ scope 2.0, the latest version of the company’s minimally invasive imaging platform. The technology was recently used for the first time in a clinical case by Kyle Anderson, MD, at Anderson Sports Medicine in Bingham Farms, Michigan.
Advertisement
Since its debut in 2019, the Arthrex NanoNeedle scope has offered surgeons a minimally invasive alternative to traditional arthroscopy. With a tiny, high-quality camera on the tip of a needle-like device, the NanoNeedle scope 2.0 requires only a small incision, which allows for minimal tissue disruption and results in a smaller scar. The high-resolution images provide surgeons the ability to diagnose and treat injuries and ailments in many parts of the body in a less disruptive way, which leads to less nerve damage1 and pain,2 and faster recovery for patients.3
The NanoNeedle scope 2.0 is the third-generation camera in the Nano product line. The new 720 × 720 sensor delivers a significant upgrade in image performance from the previous 400 × 400, offering a 224% increase in pixel resolution and more than three times the pixel density within the same compact footprint and dramatically enhanced clarity.
“As a surgeon, it’s exciting to be able to offer patients the most minimally invasive options. This feels like a fundamental shift to a less invasive way of treating some injuries,” said Dr. Anderson. “With a traditional scope, we do the procedure through an instrument that is roughly the size of a pen. Now with NanoNeedle scope 2.0, it’s as small as an IV catheter. We are at the beginning of an exciting era in sports medicine where we can be ultra-minimally invasive, because of what Nano technology can do.”
As part of the Synergy Vision™ system, the NanoNeedle scope 2.0 received U.S. Food and Drug Administration (FDA) clearance to use the technology for pediatric orthopedics. Dr. Anderson successfully completed the first case on a 13-year-old baseball player with a labrum tear, noting it’s ideal for use in pediatric patients with small, immature joints.
“This is the first arthroscopic camera device on the market that provides a sterile, minimally invasive option for pediatrics, rather than using larger instruments intended for adults,” said Arthrex Senior Product Manager Ryan Kellar. “NanoNeedle scope 2.0 allows the surgeon to use less fluid to swell the joint and keep cartilage damage to a minimum. It’s a camera that can become the gold standard in pediatric cases.”
The 1.9 mm diameter NanoNeedle scope is also used in thoracic, laparoscopic and endoscopic procedures. With the launch of the NanoNeedle scope 2.0, upgrades in visualization and performance are expected to be more widely adopted for many more procedures, both simple and complex. NanoNeedle scope 2.0 currently works with the Arthrex Synergy Vision system, but a mobile unit is in development and expected to be released later this year.
“The device is terrific, the image quality is amazing,” Dr. Anderson said. “It’s like you’re seeing the latest and greatest of traditional arthroscopy, but you’re looking through a needle that creates just a puncture wound that doesn’t even require any incisions.”
About Arthrex
Arthrex, headquartered in Naples, Florida, is a global medical device company and leader in multispecialty minimally invasive surgical technology innovation, scientific research, manufacturing and medical education. The company has pioneered the field of arthroscopy and sports medicine and develops more than 1,000 new products and related procedures annually to advance minimally invasive orthopedic surgery, trauma, spine, cardiothoracic, orthobiologics and arthroplasty innovation worldwide. Arthrex also specializes in the latest 4K multispecialty surgical visualization and OR integration technology solutions. For more information, visit Arthrex.com.
Physician is a paid consultant of Arthrex, Inc.
References
- Stornebrink T, Altink JN, Appelt D, Wijdicks CA, Stufkens SAS, Kerkhoffs GMMJ. Two-millimetre diameter operative arthroscopy of the ankle is safe and effective. Knee Surg Sports Traumatol Arthrosc. 2020;28(10):3080-3086. doi:10.1007/s00167-020-05889-7
- Schaver AL, Lash JG, MacAskill ML, et al. Partial meniscectomy using needle arthroscopy associated with significantly less pain and improved patient reported outcomes at two weeks after surgery: a comparison to standard knee arthroscopy. J Orthop. 2023;41:63-66. doi:10.1016/j.jor.2023.06.00
- Colasanti CA, Mercer NP, Garcia JV, Kerkhoffs GMMJ, Kennedy JG. In-office needle arthroscopy for the treatment of anterior ankle impingement yields high patient satisfaction with high rates of return to work and sport. Arthroscopy. 2022;38(4):1302-1311. doi:10.1016/j.arthro.2021.09.016
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/arthrex-achieves-first-case-with-nanoneedle-scope-2-0–302564590.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/arthrex-achieves-first-case-with-nanoneedle-scope-2-0–302564590.html
SOURCE Arthrex, Inc.
 Disclaimer: The above press release comes to you under an arrangement with PR Newswire. USA Newshour takes no editorial responsibility for the same.
Disclaimer: The above press release comes to you under an arrangement with PR Newswire. USA Newshour takes no editorial responsibility for the same.