CRISPR Therapies Clinical Trial Pipeline Shows Potential with Active Contributions from 25+ Key Companies | DelveInsight
CRISPR therapies offer precise gene-editing solutions for treating genetic disorders and other complex diseases. Rising prevalence of genetic conditions drives adoption, and emerging CRISPR-based therapies are expected to further expand market opportunities.
New York, USA, Oct. 02, 2025 (GLOBE NEWSWIRE) — CRISPR Therapies Clinical Trial Pipeline Shows Potential with Active Contributions from 25+ Key Companies | DelveInsight
Advertisement
CRISPR therapies offer precise gene-editing solutions for treating genetic disorders and other complex diseases. Rising prevalence of genetic conditions drives adoption, and emerging CRISPR-based therapies are expected to further expand market opportunities.
DelveInsight’s ‘CRISPR Therapies Pipeline Insight 2025‘ report provides comprehensive global coverage of pipeline CRISPR therapies in various stages of clinical development, major pharmaceutical companies are working to advance the pipeline space and future growth potential of the CRISPR therapies pipeline domain.
Key Takeaways from the CRISPR Therapies Pipeline Report
- DelveInsight’s CRISPR therapies pipeline report depicts a robust space with 25+ active players working to develop 30+ pipeline CRISPR therapies.
- Key CRISPR therapies companies, such as Locus Biosciences, Intellia Therapeutics, Inc., Caribou Biosciences, Inc., Tango Therapeutics, KSQ Therapeutics, Emendo Biotherapeutics, Beam Therapeutics, Excision BioTherapeutics, Eli Lilly and Company, Epicrispr Biotechnologies, Inc., Scribe Therapeutics, and others, are evaluating new CRISPR therapies to improve the treatment landscape.
- Promising pipeline CRISPR therapies such as LBP-EC01, NTLA-2002, CB-010, TNG260, KSQ-4279, EMD-101, BEAM-301, EBT-101, VERVE-201, EPI 331, STX-1150, and others are under different phases of CRISPR therapies clinical trials.
- In September 2025, Intellia Therapeutics, Inc. announced it had completed enrollment in the global Phase III HAELO study of lonvoguran ziclumeran (lonvo-z) for the treatment of hereditary angioedema (HAE).
- In July 2025, Eli Lilly and Company announced the successful completion of its acquisition of Verve Therapeutics, Inc. and its in vivo gene editors for up to USD 1.3 billion.
- In June 2025, Intellia Therapeutics, Inc. announced three-year follow-up data from the Phase I portion of the ongoing Phase I/II study in patients with HAE after receiving a single dose of lonvoguran ziclumeran (lonvo-z, also known as NTLA-2002).
- In January 2025, Caribou Biosciences, Inc. announced initiation of the GALLOP Phase I clinical trial evaluating CB-010 in patients with lupus nephritis (LN) and extrarenal lupus (ERL).
- In January 2025, Intellia Therapeutics, Inc. announced that the first patient had been dosed in the global Phase III study of NTLA-2002 for the treatment of hereditary angioedema (HAE).
- In September 2024, Caribou Biosciences, Inc. announced that the US Food and Drug Administration (FDA) granted Fast Track designation to CB-010 for refractory systemic lupus erythematosus (SLE).
- In August 2024, Locus Biosciences, Inc. announced positive results from Part 1 of its two-part Phase II ELIMINATE trial. This Phase II trial is evaluating LBP-EC01, a CRISPR-Cas3 genetically engineered bacteriophage therapy designed to treat patients with uncomplicated urinary tract infections (uUTIs) caused by antimicrobial-resistant (AMR) and multi-drug-resistant (MDR) Escherichia coli (E. coli).
Request a sample and discover the recent advances in CRISPR therapies @ CRISPR Therapies Pipeline Report
The CRISPR therapies pipeline report provides detailed profiles of pipeline assets, a comparative analysis of clinical and non-clinical stage CRISPR therapies drugs, inactive and dormant assets, a comprehensive assessment of driving and restraining factors, and an assessment of opportunities and risks in the CRISPR therapies clinical trial landscape.
CRISPR Therapies Overview
CRISPRs are unique DNA segments known as “clusters of regularly interspaced short palindromic repeats.” These regions are defined by two key features: repeated nucleotide sequences and intervening spacers. The repeats consist of short DNA sequences that occur multiple times, while the spacers are unique DNA fragments positioned between these repeats. CRISPRs function as part of the bacterial immune defense system.
The CRISPR-Cas9 system is valued for its precision and relative simplicity. Its specificity relies on two elements: a 20-base target sequence in the CRISPR RNA (crRNA) array and a protospacer adjacent motif (PAM) in the host genome. The Cas9 protein recognizes the PAM but cannot easily be altered to target different PAM sequences. Once introduced into cells through a plasmid, Cas9, guided by crRNA, locates and binds to the matching DNA sequence in the genome.
Casgevy represents the first FDA-approved therapy to harness CRISPR/Cas9. It involves genome editing of a patient’s hematopoietic stem cells, offering a treatment for sickle cell disease in individuals aged 12 and older with recurrent vaso-occlusive crises.
Genomes encode instructions in DNA, and genome editing alters these instructions by introducing cuts that exploit natural repair mechanisms. CRISPR-Cas9 enables this through a two-part system. The first part is Cas9, an endonuclease with two domains, RuvC and HNH, that cut opposite DNA strands, creating double-strand breaks. The second part is a single guide RNA (sgRNA) containing a scaffold sequence for Cas9 binding and a 20-base spacer complementary to the target site near a PAM. The sgRNA directs Cas9 to the precise genomic region, after which repair occurs through non-homologous end joining (NHEJ) or homology-directed repair (HDR). This system has transformed biomedical research and is now advancing into clinical applications, ushering in a new era of CRISPR-based therapies.
Despite its promise, CRISPR use raises several concerns. Off-target effects remain a challenge, with some studies showing minimal errors while others report large, unintended DNA insertions or deletions. Additionally, immune responses against CRISPR proteins may occur, as pre-existing Cas9 antibodies or Cas9-reactive T cells have been detected in some individuals. Finally, most clinical applications so far rely on ex vivo methods, where cells are edited outside the body before being reintroduced into patients.
Find out more about CRISPR therapies drugs @ CRISPR Therapies Analysis
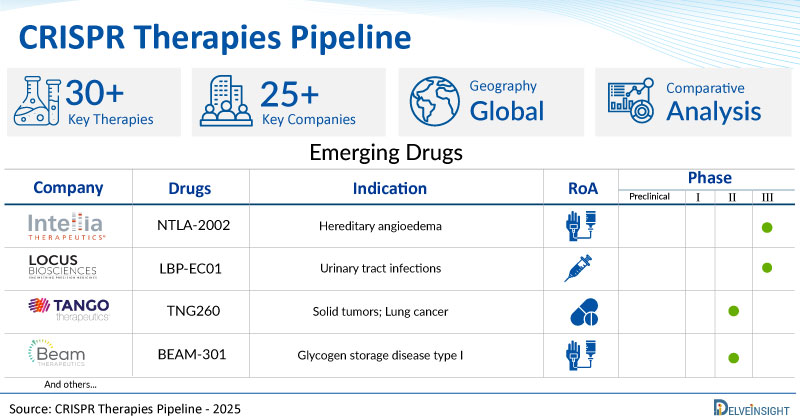
A snapshot of the Pipeline CRISPR Therapies Drugs mentioned in the report:
| Drugs | Company | Phase | Indication | RoA |
| NTLA-2002 | Intellia Therapeutics, Inc. | III | Hereditary angioedema | Intravenous |
| LBP-EC01 | Locus Biosciences | II/III | Urinary tract infections | Intraurethral |
| TNG260 | Tango Therapeutics | I/II | Solid tumors; Lung cancer | Oral |
| BEAM-301 | Beam Therapeutics | I/II | Glycogen storage disease type I | Intravenous |
| KSQ-4279 | KSQ Therapeutics | I/II | Solid tumors | Oral |
| EBT-101 | Excision BioTherapeutics | I/II | HIV-1-infection | Intravenous |
| CB-010 | Caribou Biosciences, Inc. | I | Non-Hodgkin’s lymphoma; Systemic lupus erythematosus | Infusion |
Learn more about the emerging CRISPR therapies @ CRISPR Therapies Clinical Trials
CRISPR Therapies Therapeutics Assessment
The CRISPR therapies pipeline report proffers an integral view of the emerging CRISPR therapies segmented by stage, product type, molecule type, and route of administration.
Scope of the CRISPR Therapies Pipeline Report
- Coverage: Global
- Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- Therapeutics Assessment By Route of Administration: Intravenous, Subcutaneous, Oral, Intramuscular
- Therapeutics Assessment By Molecule Type: Monoclonal antibody, Small molecule, Peptide
- Key CRISPR Therapies Companies: Locus Biosciences, Intellia Therapeutics, Inc., Caribou Biosciences, Inc., Tango Therapeutics, KSQ Therapeutics, Emendo biotherapeutics, Beam Therapeutics, Excision BioTherapeutics, Eli Lilly and Company, Epicrispr Biotechnologies, Inc., Scribe Therapeutics, and others
- Key Pipeline CRISPR Therapies: LBP-EC01, NTLA-2002, CB-010, TNG260, KSQ-4279, EMD-101, BEAM-301, EBT-101, VERVE-201, EPI 331, STX-1150, and others
Dive deep into rich insights for new CRISPR therapies, visit @ CRISPR Therapies Drugs
Table of Contents
| 1. | CRISPR Therapies Pipeline Report Introduction |
| 2. | CRISPR Therapies Pipeline Report Executive Summary |
| 3. | CRISPR Therapies Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | CRISPR Therapies Clinical Trial Therapeutics |
| 6. | CRISPR Therapies Pipeline: Late-Stage Products (Pre-registration) |
| 7. | CRISPR Therapies Pipeline: Late-Stage Products (Phase III) |
| 8. | CRISPR Therapies Pipeline: Mid-Stage Products (Phase II) |
| 9. | CRISPR Therapies Pipeline: Early-Stage Products (Phase I) |
| 10. | CRISPR Therapies Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the CRISPR Therapies Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the CRISPR Therapies Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the CRISPR therapies pipeline therapeutics, reach out @ CRISPR Therapies Therapeutics
Related Reports
CRISPR Market
CRISPR Market Size, Target Population, Competitive Landscape & Market Forecast – 2034 report deliver an in-depth understanding of the market trends, market drivers, market barriers, and key CRISPR companies, including Vertex, CRISPR, Bluebird Bio, Prevail Therapeutics, Scribe Therapeutics, Caribou Biosciences, among others.
Hereditary Angioedema Pipeline
Hereditary Angioedema Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key HAE companies, including BioCryst Pharmaceuticals, KalVista Pharmaceuticals, Pharvaris, BioMarin Pharmaceutical, Ionis Pharmaceuticals, Inc., Intellia Therapeutics, among others.
Urinary Tract Infections Pipeline
Urinary Tract Infections Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key UTIs companies, including Wockhardt, GlaxoSmithKline, Iterum Therapeutics, Spero Therapeutics, VenatoRx Pharmaceuticals, Helperby Therapeutics, Spexis, LUCA Biologics, Seed Health, Inc., Aelin Therapeutics, Omnix Medical, Inmunotek S.L., Sinovent Pty Ltd., Qilu Pharmaceuticals, Entasis Therapeutics, Adaptive Phage Therapeutics, Locus Biosciences, Nabriva Therapeutics, Utility Therapeutics, Zensun (Shanghai) Sci & Tech, Fedora Pharmaceuticals, Osel Inc., Evofem Biosciences, Enlivex, Fimbrion Therapeutics, Rebiotix, MerLion Pharmaceuticals, Allecra Therapeutics, Lakewood Amedex, Sumitomo Dainippon Pharma, Sihuan Pharmaceuticals, SuperTrans Medical, Asieris Pharmaceuticals, among others.
Complicated Urinary Tract Infections Pipeline
Complicated Urinary Tract Infections Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key cUTIs companies, including Spero Therapeutics, Jiangsu Hengrui Medicine Co., Everest Medicines, NeuroRx, Inc., Pfizer, among others.
Systemic Lupus Erythematosus Pipeline
Systemic Lupus Erythematosus Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key SLE companies, including Biogen, Idorsia Pharmaceuticals, AbbVie, Biosenic, Roche, Eisai, Daiichi Sankyo Company, Carna Bioscience, Asahi Kasei Pharma, Sanofi, Alpine Immune Sciences, Novartis Pharmaceuticals, Sana Biotechnology, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Pharmaceutical Consulting Services
Healthcare Conference Coverage
Pipeline Assessment
Healthcare Licensing Services
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant, and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance.
CONTACT: Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com
 Disclaimer: The above press release comes to you under an arrangement with GlobeNewswire. USA Newshour takes no editorial responsibility for the same.
Disclaimer: The above press release comes to you under an arrangement with GlobeNewswire. USA Newshour takes no editorial responsibility for the same.
