EU regulatory body trust AstraZeneca’s COVID-19 vaccine amid lot of safety concerns
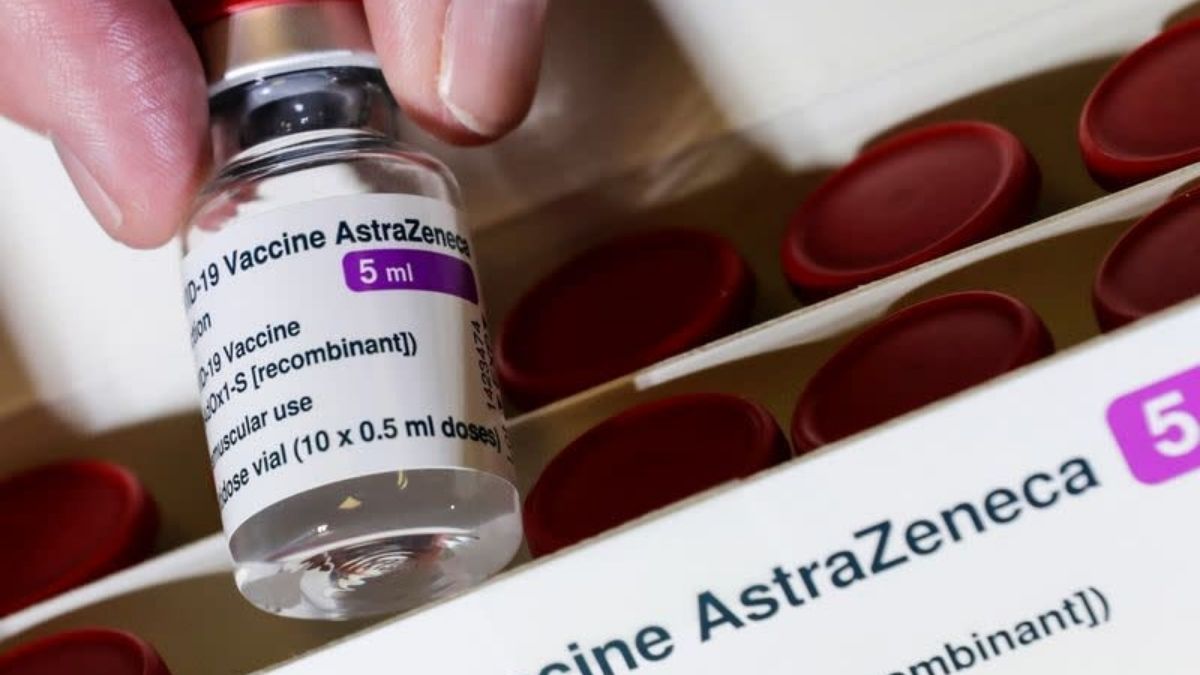
AstraZeneca’s COVID-19 vaccine efficacy is still upheld by The EU’s drug regulator body even after the vaccine faced an order for investigation after more than a dozen nations suspend its use over the risks following an investigation into reports of blood disorders.
Following are reactions after the European Medicines Agency (EMA) proffered an update on its views over the Oxford-AstraZeneca vaccine.
Advertisement
On the information available, it is a good general stance taken by EMA, Bomsel said. “A restart in vaccinations with this vaccine will be a positive signal for the public.”
The past damage to communication disaster is unrepairable as per EMA’s verdict so they could not make any possible communication to repair what the governments have done.
“On the other hand, EMA has always said that vaccination should not be interrupted. It is the governments that have made political and emotional decisions. The important thing is that the EMA today reiterated what it has always said, and which is also what all scientists except a few German scientists have always said: that there is not the slightest significant percentage of cases of thrombosis, and that the vaccine is safe.
EMA also cleared a lot of illusions making round such as the possibility that these rare events may be due to previous infections and not to the vaccine.”
